Efficacy and Improved Resistance Potential of a Cofactor-Independent InhA Inhibitor of Mycobacterium tuberculosis in the C3HeB/FeJ Mouse Model | Antimicrobial Agents and Chemotherapy

Isonicotinylation is a histone mark induced by the anti-tuberculosis first-line drug isoniazid | Nature Communications

NOS2-deficient mice with hypoxic necrotizing lung lesions predict outcomes of tuberculosis chemotherapy in humans | Scientific Reports

Mycobacterium tuberculosis persistence mutants identified by screening in isoniazid-treated mice | PNAS

Direct Oxidation and Covalent Binding of Isoniazid to Rodent Liver and Human Hepatic Microsomes: Humans Are More Like Mice than Rats | Chemical Research in Toxicology

JCI Insight - Interleukin-17 limits hypoxia-inducible factor 1α and development of hypoxic granulomas during tuberculosis

A Physiologically Based Pharmacokinetic Model of Isoniazid and Its Application in Individualizing Tuberculosis Chemotherapy | Antimicrobial Agents and Chemotherapy

Effect of niacin supplementation on nausea-like behaviour in an isoniazid-induced mouse model of pellagra | British Journal of Nutrition | Cambridge Core

pH‐Responsive Isoniazid‐Loaded Nanoparticles Markedly Improve Tuberculosis Treatment in Mice - Hwang - 2015 - Small - Wiley Online Library

Isonicotinylation is a histone mark induced by the anti-tuberculosis first-line drug isoniazid | Nature Communications

Efficacy and Improved Resistance Potential of a Cofactor-Independent InhA Inhibitor of Mycobacterium tuberculosis in the C3HeB/FeJ Mouse Model | Antimicrobial Agents and Chemotherapy

Epidemiology in History Ficacy, Effectiveness, Toxicity, Durability, and Duration | PDF | Tuberculosis | Hiv/Aids
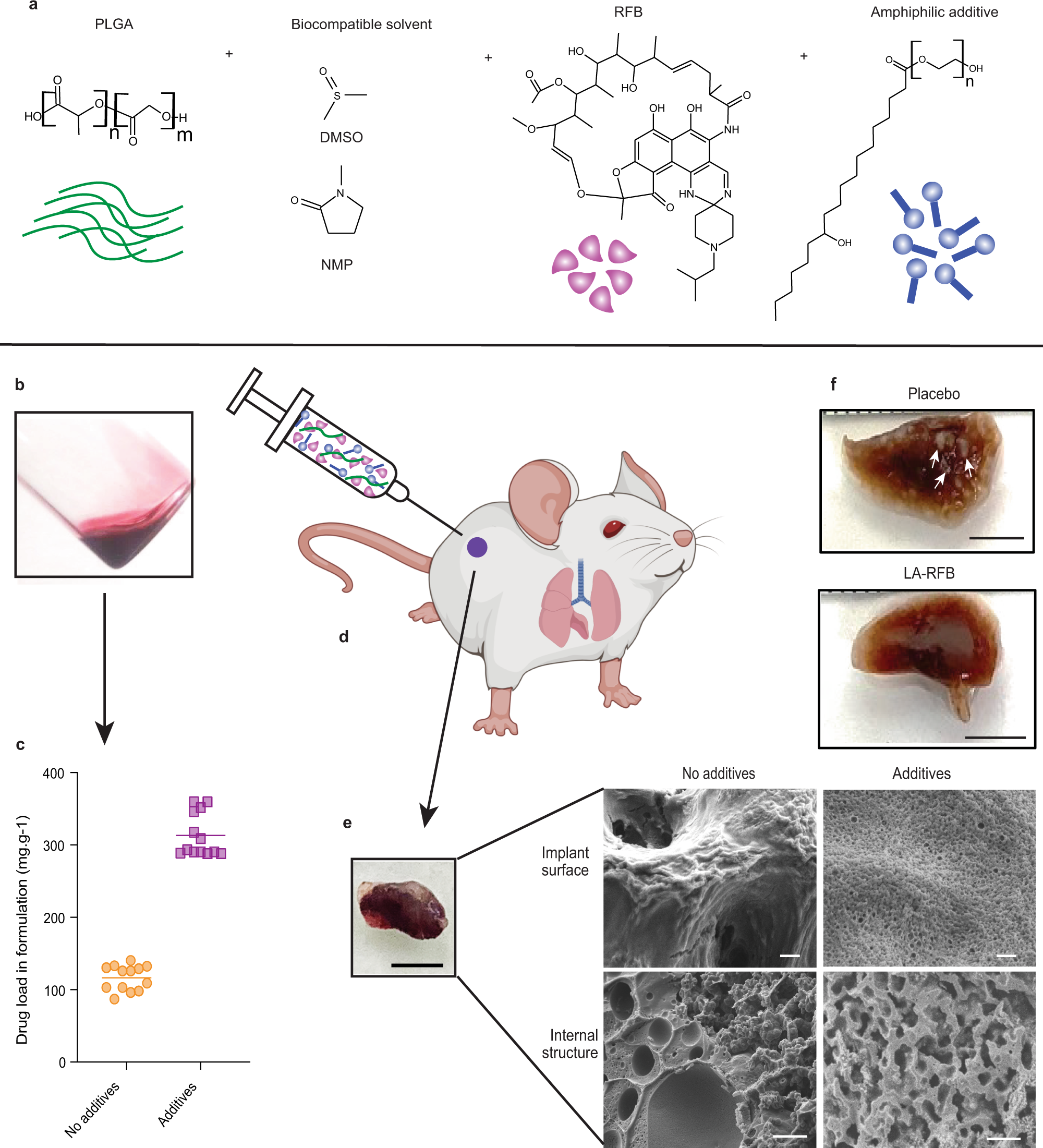
A long-acting formulation of rifabutin is effective for prevention and treatment of Mycobacterium tuberculosis | Nature Communications

Mycobacterium tuberculosis persistence mutants identified by screening in isoniazid-treated mice | PNAS

Hepatoprotective effects of Malus hupehensis tea against isoniazid- and rifampicin-induced liver injury by regulating cytochrome P450 in mice - ScienceDirect

Treatment of Tuberculosis Using a Combination of Sustained-Release Rifampin-Loaded Microspheres and Oral Dosing with Isoniazid | Antimicrobial Agents and Chemotherapy

Direct Oxidation and Covalent Binding of Isoniazid to Rodent Liver and Human Hepatic Microsomes: Humans Are More Like Mice than Rats | Chemical Research in Toxicology

Direct Oxidation and Covalent Binding of Isoniazid to Rodent Liver and Human Hepatic Microsomes: Humans Are More Like Mice than Rats | Chemical Research in Toxicology

A Physiologically Based Pharmacokinetic Model of Isoniazid and Its Application in Individualizing Tuberculosis Chemotherapy | Antimicrobial Agents and Chemotherapy

Assessing whether isoniazid is essential during the first 14 days of tuberculosis therapy: a phase 2a, open-label, randomised controlled trial - ScienceDirect