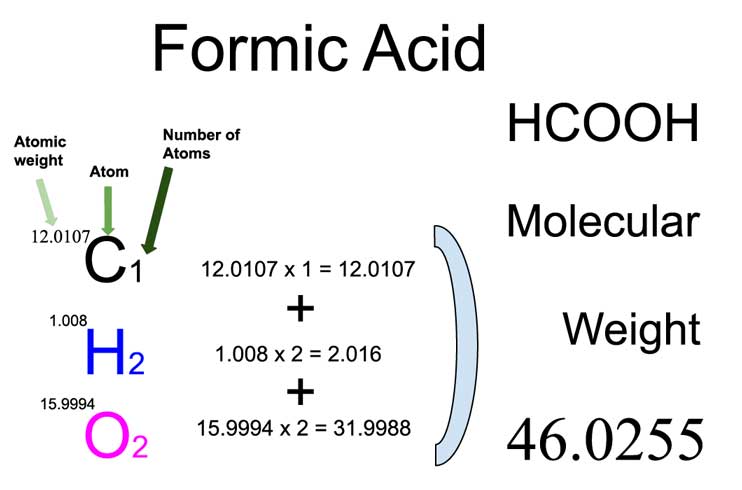
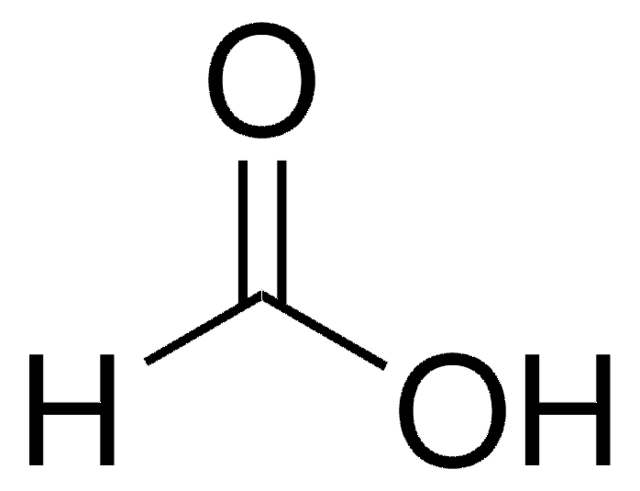
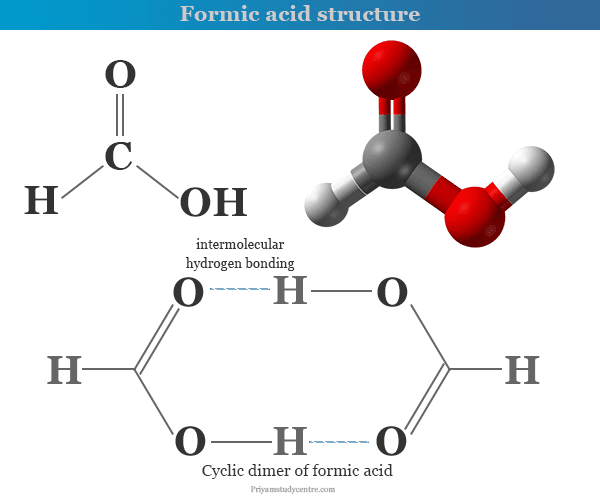
Formic Acid (HCOOH) - Structure, Molecular Mass, physical Properties, chemical properties, Uses and FAQs of formic acid (HCOOH)

Reaction and separation system for CO2 hydrogenation to formic acid catalyzed by iridium immobilized on solid phosphines under base-free condition - ScienceDirect

Dissociation constant (K_a) of formic acid and acetic acid are 2.5xx10^-4 and 0.5xx10^-5 respect... - YouTube

When a solution of formic acid was titrated with KOH solution, the pH of the solution was 3.65 when half the acid was neutralized. Calculate Ka(HCOOH) .
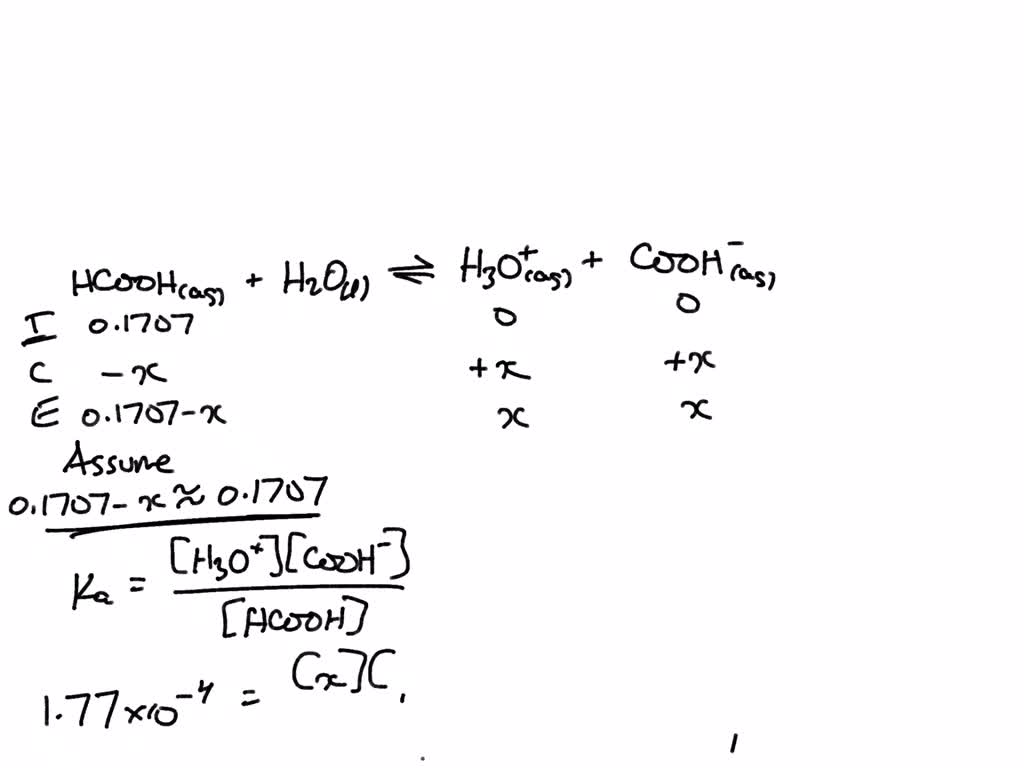
SOLVED: Formic acid is a weak acid with the formula HCOOH; the value of Ka for formic acid is 1.77 x 10-4 In aqueous solution, formic acid partially dissociates according to the

The Ka values of formic acid and acetic acid are respectively 1.77 × 10^-4 and 1.75 × 10^-5 . The ratio of the acid strength of 0.1M acid is:
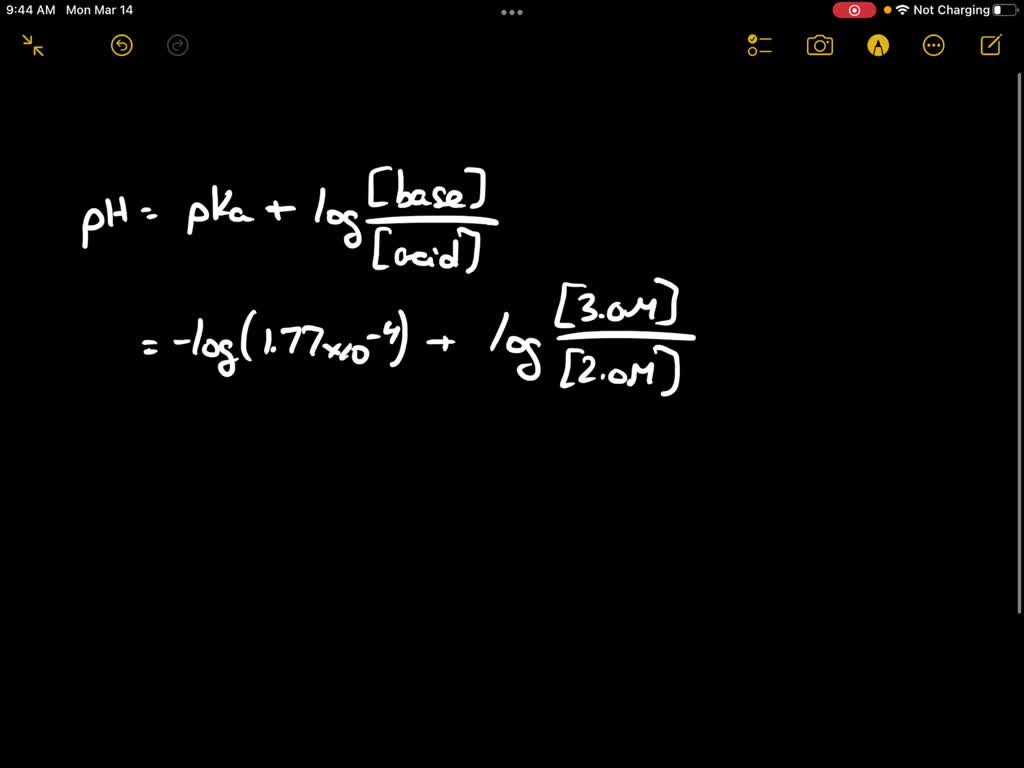
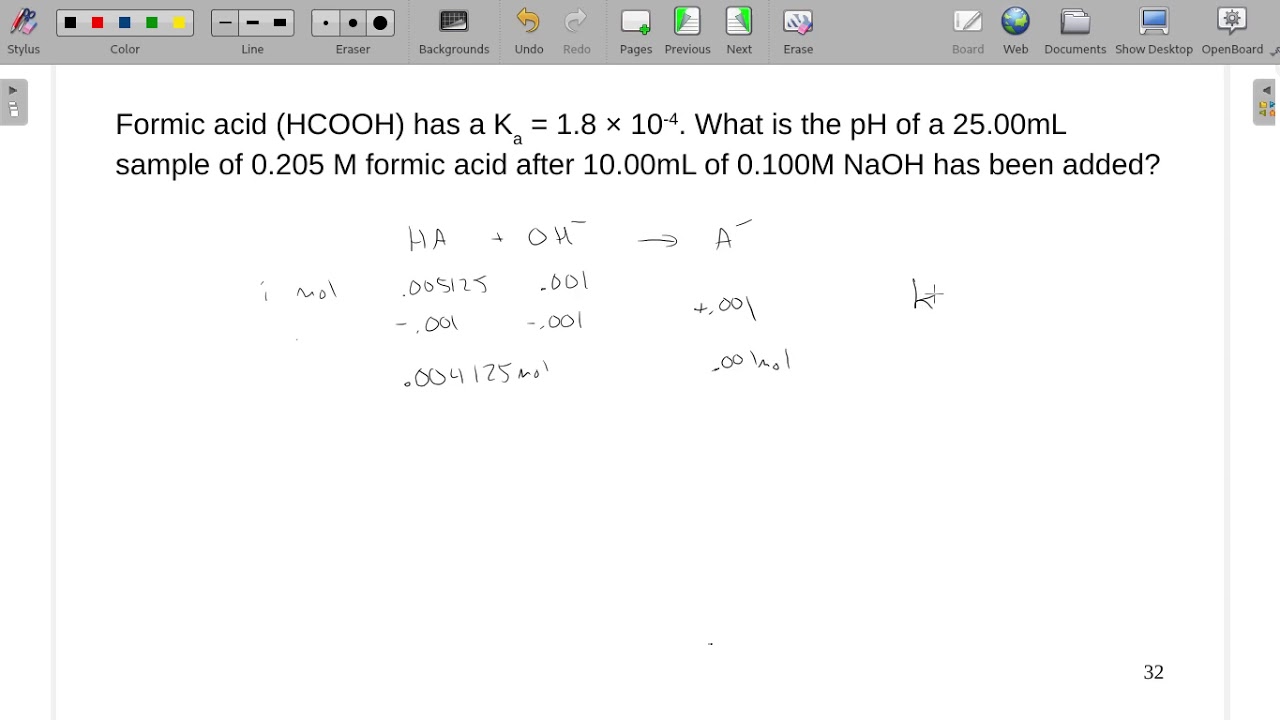
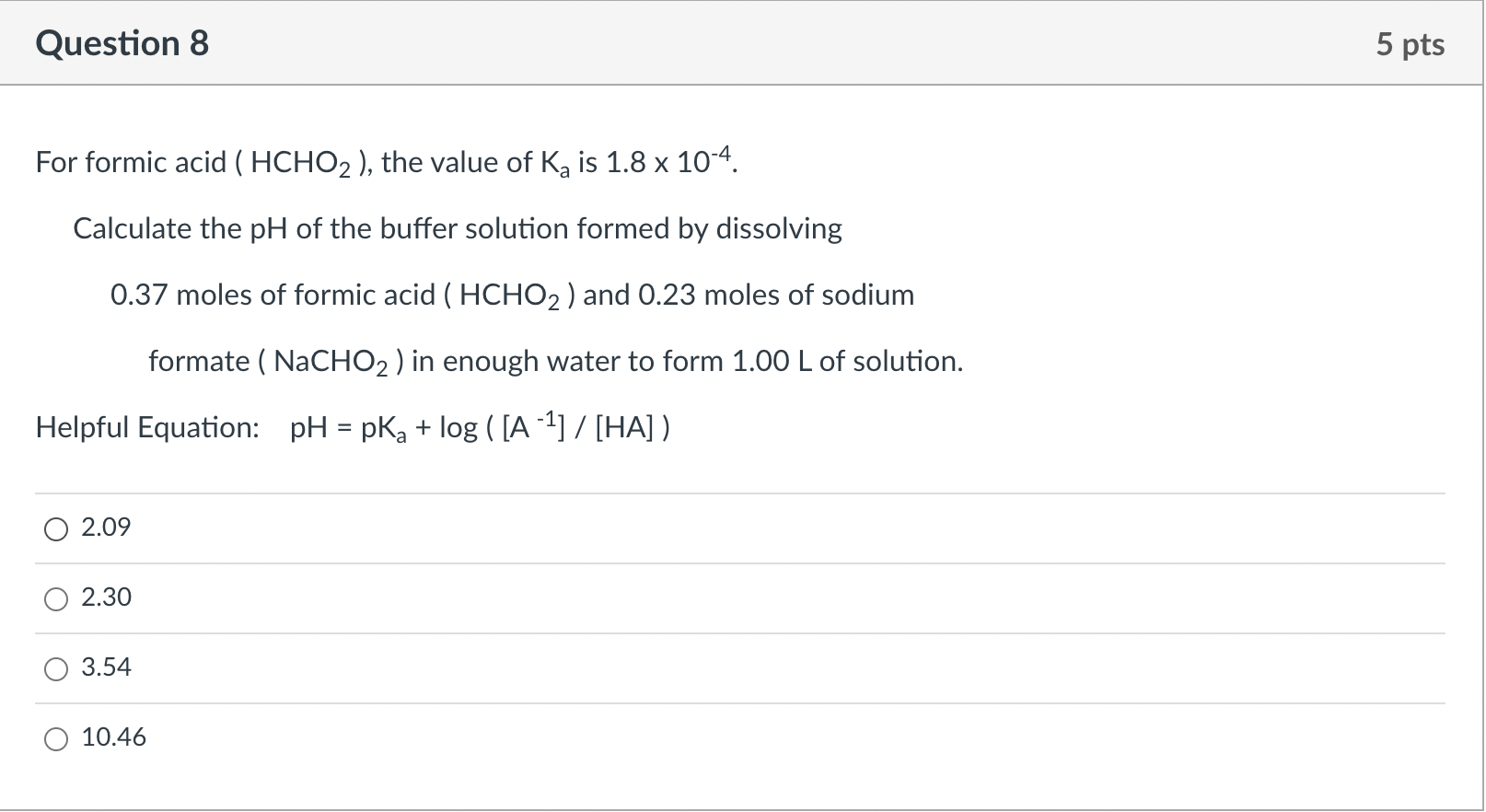
SOLVED: The Ka for formic acid, HCOOH, is 1.77 * 10^-4. HCOOH (aq) + H2O (l) ⇌ HCOO- (aq) + H3O+ (aq) What is the pH of a buffer made from 2.0

Formic Acid as Carbon Monoxide Source in the Palladium-Catalyzed N-Heterocyclization of o-Nitrostyrenes to Indoles | The Journal of Organic Chemistry

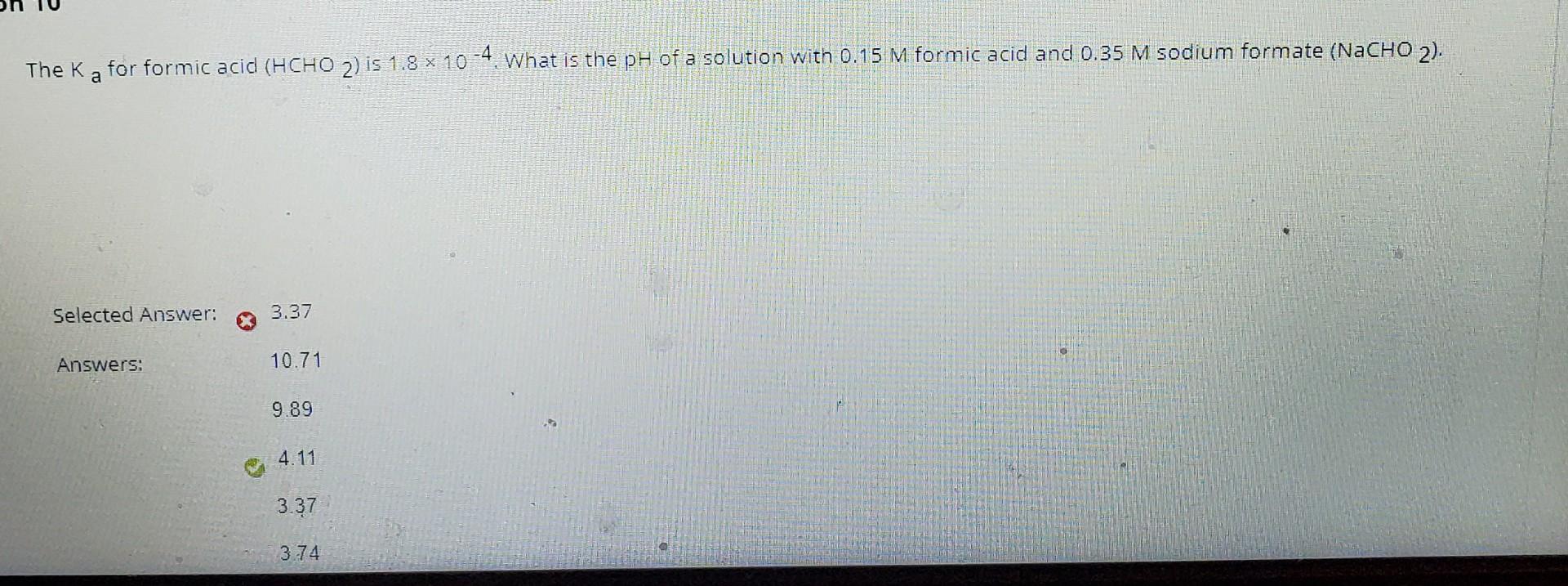
What is the pH of a 0.15 M solution of formic acid, HCOOH ? `{:("Formic Acid ",K_a),(HCOOH - YouTube

SOLVED: The value of Ka for formic acid HCOOH is 1.80x10^-4. Write the equation for the reaction that goes with this equilibrium constant: (Use H2O instead of H+.)
Formic acid, 50 ml, glass, 50 ml, CAS No. 64-18-6 | Starting material for eluent mixtures | Eluent additives for LC-MS | LC-MS | Liquid chromatography (LC, HPLC, LC-MS) | Chromatography | Applications | Carl Roth - International